Which of the Following Electron Configurations Represent an Excited State
Recall that an excited state can be identified by. This is the lower energy state which is termed as stable state.
Solved 2 Which Of The Following Electron Configurations Chegg Com
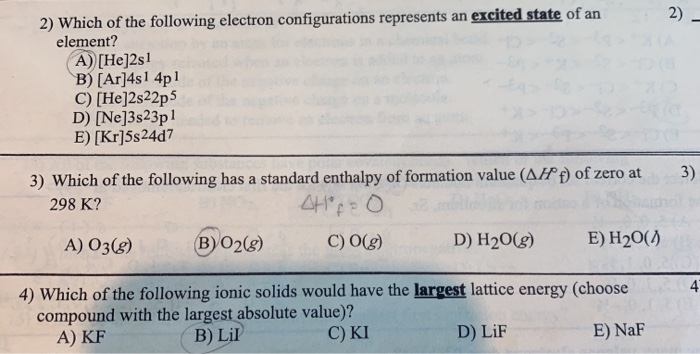
1s2 2s2 2p6 B N.

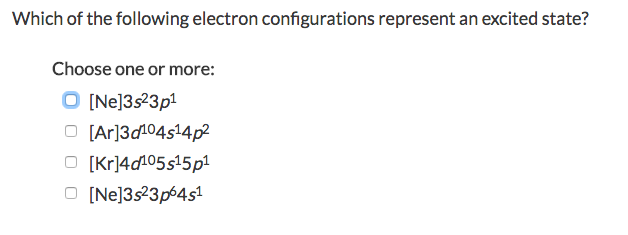
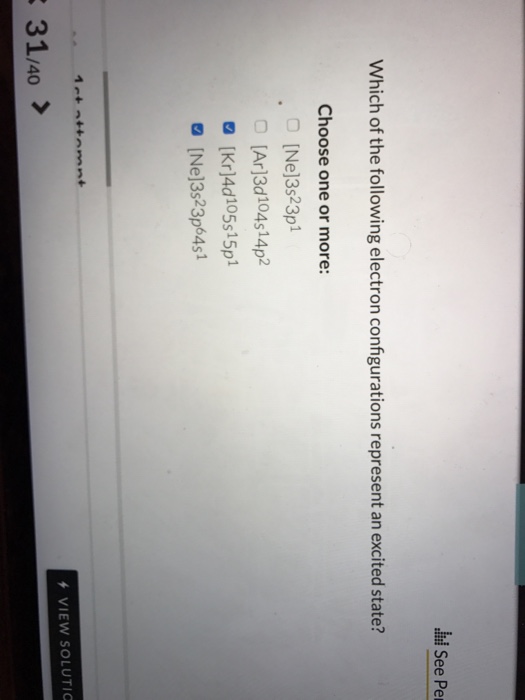
. Ne3s23p1 Ar3d104s14p2 Kr4d105s15p1 Ne3s23p64s1. Which of the following electron configurations represents an excited state of the indicated atom. An atom is said to be in an excited state if it gains energy and move to an higher energy level.
Consider the following neutral electron configurations in which n has a constant value. It may represent an excited-state electron configuration of a Mg atom Which of the following may represent an excited-state electron configuration for a cobalt atom. Which of the following electron configurations represents an excited state of the indicated atom.
The electron configuration that represent an excited state for an atom of calcium is 2 8 7 3. One electron is transferred from 3s to 3p level to form excited state. Ne3s23p1 Ar3d104514p2 kr4d105s15p1 Ne3s23p6451.
See Periodic Table See Hint Which of the following electron configurations represent an excited state. Dont run the numbers together. 1s2 2s2 2p3 C P.
Which of the following electron configurations of neutral atoms represent excited states. This is the upper energy state and is termed as the unstable state. The following electron configurations represent excited states.
Identify the element and write its ground-state condensed electron configuration. An excited state differs from a ground state which is when all of the atoms electrons are in the their lowest possible orbital. So any electron configuration in which the last electron again the valence electron is in a higher energy orbital this element is said to be in an excited state.
Which of the following electron configurations of neutral atoms represent excited states. Type your answer using the format Ar 4s2 3d10 4p2 for Ar4s23d104p2. If the element were to become excited the electron could.
The given electron configuration is in its excited state. See Periodic Table See Hint Which of the following electron configurations represent an excited state. There are 2 states classified under energy levels.
Which of the following electron configurations of neutral atoms represent excited states. Xe6s24f1 2s2 1s22s22p63s23p63d2 Ar4s23d3 Kr5s14d5. Xe6s24f1 2s2 1s22s22p63s23p63d2 Ar4s23d3 Kr5s14d5.
See the answer See the answer done loading. Calcium atom has an atomic number of 20 and its electronic configuration is 2 8 8 2. See the answer.
Xe6s24f1 2s2 1s22s22p63s23p63d2 Ar4s23d3 Kr5s14d5 Garcia Nov 11 2012 Add spaces to make your problem more readable. Electron that has skipped orbitals. Mg has atomic number of 12.
Choose one or more. 2 2s2 3 1s2 2s2 2p6 3s2 3p6 3d2 44s2 3d3 55s1 4d5. The orbitals can contain a maximum number of electrons.
Choose one or more. The electronic configuration of the atom that represents excited state is P. Choose one or more.
1 s 2 2 s 2 2 p 6 3 s 1 3 p 1 represents an atom of magnesium in an excited state. Which of the following electron configurations of neutral atoms represent excited states. Write the ground state electron configurations of the following ions.
For example if we look at the ground state electrons in the energetically lowest available orbital of oxygen the electron configuration is 1s2 2s2 2p4. All the electrons which are present in this state always come. Which of the following electron configurations represent an excited state.
Orbitals that are not completely filled and the next one is already filled. Which of the following electron configurations I-IV represents an excited state. An excited state electron configuration refers to an atom with electrons at a higher energy level than is necessary.
For example an atom in an excited state may contain two electrons in its 1s orbital one electron in its 2s orbital and one electron. 1s2 2s2 2p6 3s2 3p2 4s1. Xe6s24f1 2s2 1s22s22p63s23p63d2 Ar4s23d3 Kr5s14d5 CHEM.
Thus it will have 12 electrons. The ground state electronic configuration is 1 s 2 2 s 2 2 p 6 3 s 2. 1s2 2s2 2p6 3s2 3p2 4s1 Two elements that have the same ground-state valence shell configuration of ns2np2 are.
Ls2 2s2 3p2 4p1.
Solved Which Of The Following Electron Configurations Chegg Com

Solved Which Of The Following Electron Configurations Chegg Com
Solved Which Of The Following Electron Configurations Chegg Com
No comments for "Which of the Following Electron Configurations Represent an Excited State"
Post a Comment